Drug Development Services (Starting Material, Intermediate & APIs) | CDMO Services
Contract research, development, and manufacturing services organization (CDMO)
Chemveda’s CDMO (API, Starting Material and Intermediate development and manufacturing) business division covers the following departments:
- Process R&D services
- Custom chemical development services
- DoE, QbD and process safety hazard assessment
- GMP manufacturing services
- Analytical development services
- Stability studies
- Regulatory support
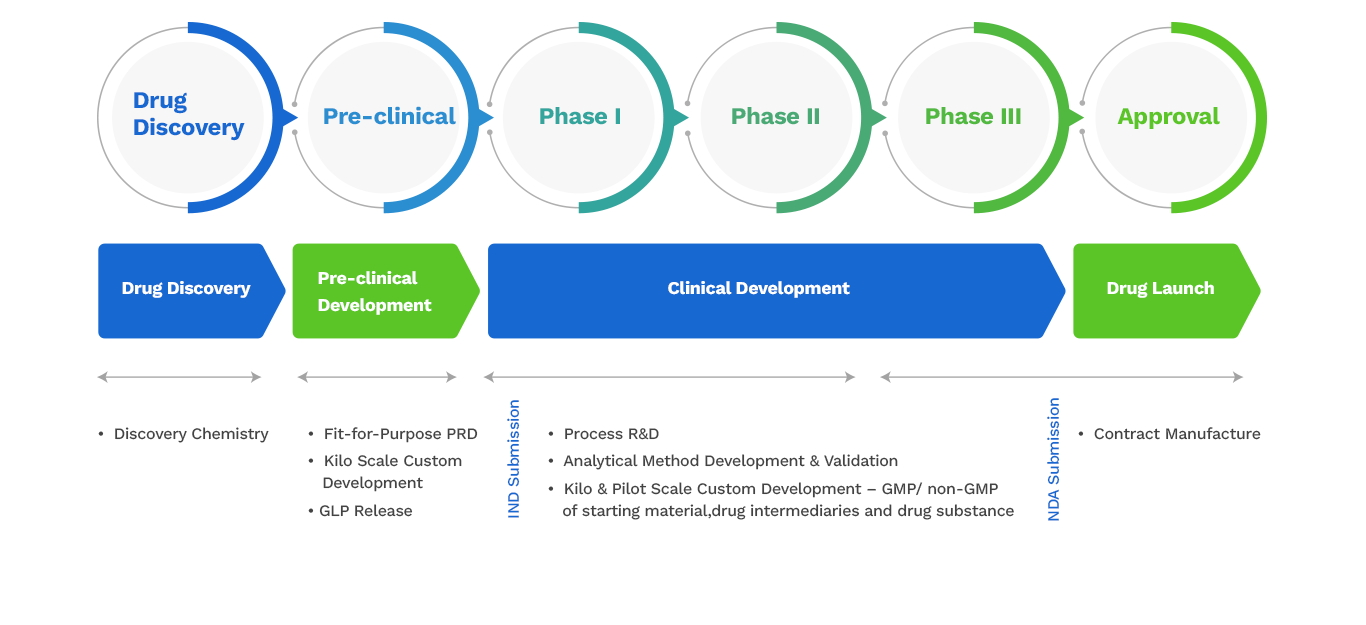
In terms of deliveries, CDMO covers the following modalities from gram-scale synthesis To multi-kilogram scale manufacturing:
- Pre-clinical, early phase, and late phase clinical development
- Regulatory starting materials (RSMs)
- Starting material development and Manufacturing
- Intermediate development & manufacturing
- New Chemical Entities (NCEs) and Active Pharmaceutical Ingredients (APIs)
- GalNAc Ligands (route scouting, selection & scaleup)
- Fine Chemical Development and Manufacturing
- Specialty Chemical Development and Manufacturing
Process R&D Services
At Chemveda Lifesciences, the process development group adopts a delivery-oriented, risk-based approach to make critical decisions right from route scout to process development, and then manufacturing. The key principles that outline each project here are: accuracy, efficiency, and speed. The overall team is comprised of chemists, engineers, and process analysts with expertise in every phase of process research.
The team works in coherence with all the other cross functional teams like process development scientists, analytical development, EHS, commercial sourcing, quality assurance and tech-transfer, etc. to ensure an on-time in full delivery.
We are equipped to follow both traditional and enhanced approaches by using ICH Q9 & 11 guidelines and implement QbD elements for finding the best operational ranges, wherever necessary. The team has expertise in solid state chemistry where we diligently optimize the process for the desired polymorph and particle size. Also, the team is well-versed with diversified requirements of phase I, II, and III, involving both scientific and regulatory aspects.
Our critical assessment for the selection of the most suitable route suitable for commercial manufacturing is based on the following MUST criteria:
- No column purifications
- No safety barriers
- IP, and regulatory implications
- Improving the cost efficiency,
- Commercial availability of raw materials and confidence in supply of materials at large scales,
- Scalability of the process, etc.
Process R&D capabilities
- Synthetic route development/route scouting
- Process development, optimization and verification
- Process hazard analysis/process safety assessment
- Pilot scale development (based on requirement)
- Technology transfer and scale-up
- Polymorph, salt, and hydrate screening
- Particle size development, and optimization
- Metabolite and impurity synthesis
- N-Acetylgalactosamine (GalNAc) oligonucleotide conjugate synthesis
- Analytical method development, and validation
- Analytical method verification, and transfer
- Stability studies, and storage
- Forced degradation studies
- Impurities identification, isolation, and characterization
- Reference and working standards qualification
- Elemental/metal analysis
Route Scouting Services
In pre-clinical development, often, the speed of delivery takes precedence over having an optimal synthetic route. However, while taking the project to the clinic an alternative synthetic route is required to deal with safety challenges and improving cost efficiency. Hence, Chemveda performs route scouting and route comparison studies to come up with more efficient synthetic route(s) involving reduced number of process steps, less expensive raw materials, safety aspects, etc. This is followed by route familiarization studies to identify the most appropriate one considering the delivery timelines and costs involved. We also specialize in route scouting of N-Acetylgalactosamine (GalNAc) ligands for oligonucleotide conjugates.
Analytical Development Services
Chemveda’s analytical development team and process scientists have a seamless co-ordination in terms of devising analytical methods to support the development of intermediates, and drug substances. The team also routinely performs method validations, stability testing of intermediates, and drug substances.
Technology Transfer Services
Our experienced Technology Absorption & Transfer Team (TATT) undertakes transfer of technology from R&D to manufacturing site or from your organization to Chemveda. The team has a proven track record of successfully taking lab processes to the manufacturing plant and deliver required quantity of chemical substances with desired specifications and within agreed delivery timelines.
Custom Chemical Development and Manufacturing Services
We have an ably equipped kilo lab facility suitable to deliver multiple batches of up to kilogram scale under GMP/non-GMP conditions.
Kilo-Lab
Chemveda’s fully-equipped kilo-lab allows for rapid scale-ups, and development of scalable & robust chemical processes using advanced process methodologies to provide a quick supply of starting material, intermediate and API in the early phase of the development
Key Aspects:
- First scale-up out of laboratory
- Rapid scale-up to support client’s initial drug testing, i.e. pre-clinical studies or sometimes phase-I of clinical trials
- Kilo gram scale deliveries
- Developing knowledge on process, and mini-piloting
- Scale-down studies to explore process changes
- Demo batches for process simulation studies
- Synthesis of quantities usually ranging from ~500g to under 10 kg


